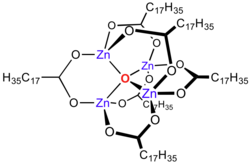
Zinc Stearate

Zinc stearate is a “zinc soap” that is widely used industrially. In this context, soap is used in its formal sense, a metal salt of a fatty acid: in this case stearic acid. It is a white solid that repels water. It is insoluble in polar solvents such as alcohol and ether but soluble in aromatic hydrocarbons (e.g., benzene) and chlorinated hydrocarbons when heated. It is the most powerful mold release agent among all metal soaps. It contains no electrolyte and has a hydrophobic effect. Its main application areas are the plastics and rubber industry, where it is used as a releasing agent and lubricant which can be easily incorporated.
Zinc carboxylates, e.g. basic zinc acetate, adopt complex formulas, and are not simply dicarboxylates of zinc. Instead the formula for most zinc carboxylates is Zn4O(O2CR)6, consisting of a Zn4O6+ core with carboxylate ligands spanning the edges.
Application
“It is widely used as a release agent for the production of many kinds of objects rubber, polyurethane, polyester processing system, powder metallurgy. These applications exploit its “”non-stick”” properties.[2] In cosmetics, zinc stearate is a lubricant and thickening to improve texture.[3]
It is an “”activator”” for rubber vulcanization by sulfur and accelerators. As discovered in the early days of vulcanization, zinc has a beneficial effect on the reaction of the sulfur with the polyolefin. The stearate is a form of zinc that is highly soluble in the nonpolar medium of the polyolefins.”
| Chemical formula | C36H70O4Zn |
|---|---|
| Molar mass | 632.33 g·mol−1 |
| Appearance | soft, white powder |
| Odor | slight, characteristic[1] |
| Density | 1.095 g/cm3, solid |
| Melting point | 120 to 130 °C (248 to 266 °F; 393 to 403 K) |
| Boiling point | decomposes |
| Solubility in water | insoluble |
| Solubility in Ethanol | insoluble |
| Solubility in ether | insoluble |
| Solubility in benzene | slightly soluble |


Reviews
There are no reviews yet.