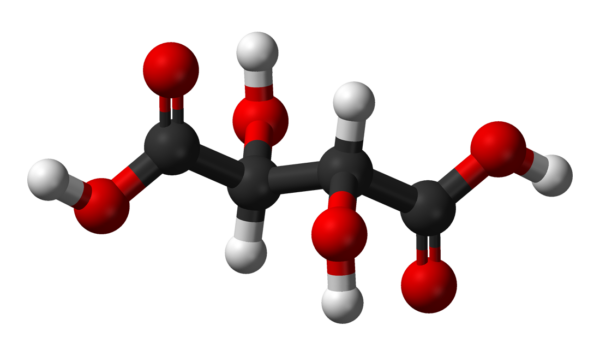
Tartaric Acid
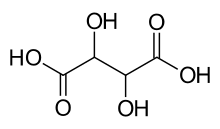
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus.[4] Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics, and is a dihydroxyl derivative of succinic acid.
Application
| Chemical formula | C4H6O6 (basic formula) |
|---|---|
| Molar mass | 150.087 g/mol |
| Appearance | White powder |
| Density | 1.737 g/cm3 (R,R- and S,S-) |
| Melting point | 169, 172 °C (R,R- and S,S-) |
| Solubility in water | 1.33 kg/L (L or D-tartaric) |
| Acidity (pKa) | L(+) 25 °C : |
| Conjugate base | Bitartrate |
| Magnetic susceptibility (χ) | −67.5·10−6 cm3/mol |


Reviews
There are no reviews yet.