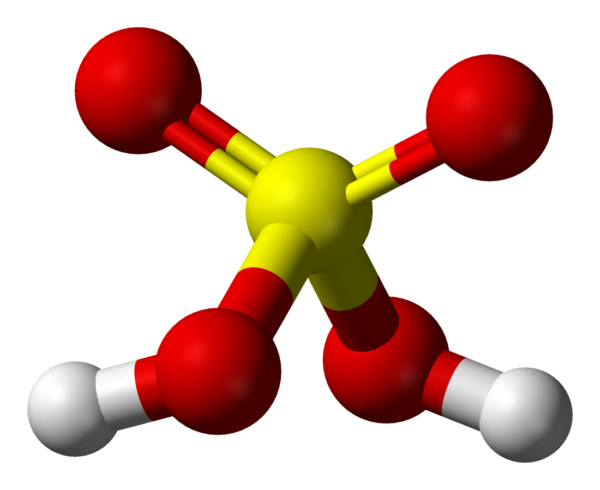
Sulphuric Acid
Pure sulfuric acid does not occur naturally due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air.[6] Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus, the reverse procedure of adding water to the acid should not be performed since the heat released may boil the solution, spraying droplets of hot acid during the process. Upon contact with body tissue, sulfuric acid can cause severe acidic chemical burns and even secondary thermal burns due to dehydration. Dilute sulfuric acid is substantially less hazardous without the oxidative and dehydrating properties; however, it should still be handled with care for its acidity.
Application
used in chemical industry for production of detergents, synthetic resins, dyestuffs, pharmaceuticals, petroleum catalysts, insecticides and antifreeze, as well as in various processes such as oil well acidicizing, aluminium reduction, paper sizing, water treatment. About 6% of uses are related to pigments and include paints, enamels, printing inks, coated fabrics and paper, and the rest is dispersed into a multitude of applications such as production of explosives, cellophane, acetate and viscose textiles, lubricants, non-ferrous metals and batteries.
| Chemical formula | H2SO4, sometimes expressed (HO)2SO2 |
|---|---|
| Molar mass | 98.079 g/mol |
| Appearance | Colourless viscous liquid |
| Odor | Odorless |
| Density | 1.8302 g/cm3, liquid |
| Melting point | 10.31[1] °C (50.56 °F; 283.46 K) |
| Boiling point | 337[1] °C (639 °F; 610 K) |
| Solubility in water | miscible, exothermic |
| Vapor pressure | 0.001 mmHg (20 °C) |
| Acidity (pKa) | pKa1 = −2.8 |
| Conjugate base | Bisulfate |
| Viscosity | Bisulfate |


Reviews
There are no reviews yet.