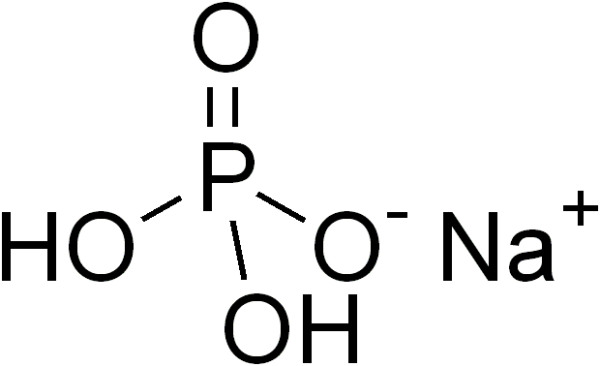
Sodium Phosphate

Sodium phosphates are popular in commerce in part because they are inexpensive and because they are nontoxic at normal levels of consumption. However, oral sodium phosphates when taken at high doses for bowel preparation for colonoscopy may in some individuals carry a risk of kidney injury under the form of phosphate nephropathy. There are several oral phosphate formulations which are prepared extemporaneously. Oral phosphate prep drugs have been withdrawn in the United States, although evidence of causality is equivocal. Since safe and effective replacements for phosphate purgatives are available, several medical authorities have recommended general disuse of oral phosphates.
Application
“Metal finishing: pH control in acid-type metal cleaners.Water Treatment, Detergents, Textiles, Oil drilling mud. Drilling mud treatment – Dispersing agent, Hardness tabilization. Cheese procssing: Its emulsfying qualities ensure an even distribution of fat and allow an easy melt. Preserve egg yolk color before refrigeration Acidulate in dry powder beverages Gelling accelerator in instant puddings .
| Sodium Phosphate | Na3PO4 |
|---|---|
| Density | 1.62 g/cm³ |
| Molecular Weight/ Molar Mass | 163.94 g/mol |
| Boiling Point | 100 °C |
| Melting Point | 1,583 °C |
| Chemical Formula | Na3PO4 |


Reviews
There are no reviews yet.