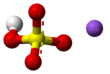
Sodium Bi Sulphate
![]()
Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO4. Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base, typically in the form of either sodium hydroxide (lye) or sodium chloride (table salt). It is a dry granular product that can be safely shipped and stored. The anhydrous form is hygroscopic. Solutions of sodium bisulfate are acidic, with a 1M solution having a pH of slightly below 1.
Application
Sodium bisulfate is used in toilet bowl cleaners and dishwasher cleaning products.Commonly used to control pH, it’s found in aquarium and swimming pool products
| Chemical formula | NaHSO4 |
|---|---|
| Molar mass | 120.06 g/mol (anhydrous) |
| Appearance | white solid |
| Density | 2.742 g/cm3 (anhydrous) |
| Melting Point | 58.5 °C (137.3 °F; 331.6 K) (monohydrate) |
| Boiling Point | decomposes to Na2S2O7 (+ H2O) at 315 °C (599 °F; 588 K) |
| Solubility in water | 28.5 g/100 mL (25 °C) |
| Solubility | Insoluble in ammonia |
| Acidity (pKa) | 1.99 |


Reviews
There are no reviews yet.