SHMP (SODIUM HEXAMETAPHOSPHATE)

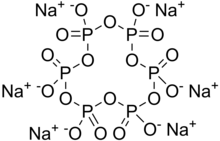
Sodium hexametaphosphate (SHMP) is a salt of composition Na6[(PO3)6]. Sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: NaPO3), of which the hexamer is one, and is usually the compound referred to by this name. Such a mixture is more correctly termed sodium polymetaphosphate. They are white solids that dissolve in water.
SHMP is used as a sequestrant and has applications within a wide variety of industries, including as a food additive in which it is used under the E number E452i. Sodium carbonate is sometimes added to SHMP to raise the pH to 8.0–8.6, which produces a number of SHMP products used for water softening and detergents.
Application
Hexametaphosphate is used as sequestering agent. It is used in the industry of soap, detergents, water treatment, metal finishing and plating, pulp and paper manufacture, synthesis of polymers, photographic products, textiles, scale removal and agriculture. The salt forms of phosphate polymers is used as a sequestering agent. As phosphate polymers themselves are hydrated in water at high temperature or high pH, and thereby revert to a more simple and stable phosphate form, which can no longer sequester metal ions.
| Chemical formula | Na6P6O18 |
|---|---|
| Molar mass | 611.7704 g mol−1 |
| Appearance | White crystals |
| Odor | odorless |
| Density | 2.484 g/cm3 |
| Melting point | 628 °C (1,162 °F; 901 K) |
| Boiling point | 1,500 °C (2,730 °F; 1,770 K) |
| Solubility in water | soluble |
| Solubility | insoluble in organic solvents |
| Refractive index (nD) | 1.482 |


Reviews
There are no reviews yet.