Red Oxide
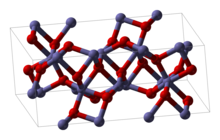
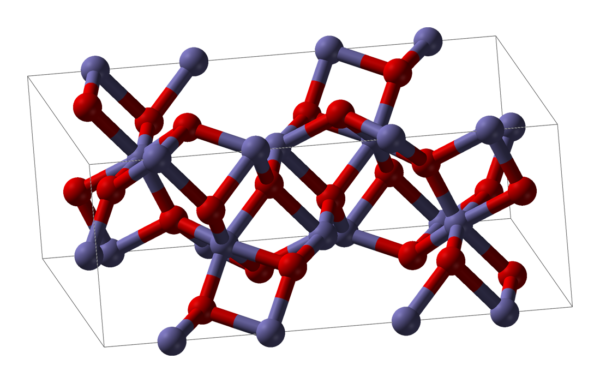
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as Hydrous ferric oxide.
Application
It is used for colour washes, distemper and oil paints. It is also used in making coloured paper. Red oxide pigments are widely used as primers for painting structural steel, automobile bodies, ship bottoms, etc.
| Chemical formula | Fe2O3 |
|---|---|
| Molar mass | 159.687 g·mol−1 |
| Appearance | Red solid |
| Odor | Odorless |
| Density | 5.25 g/cm3 |
| Melting point | 1,539 °C (2,802 °F; 1,812 K)[1] |
| Solubility in water | Insoluble |
| Solubility | Soluble in diluted acids,[1] barely soluble in sugar solution[2] |
| Magnetic susceptibility (χ) | +3586.0×10−6 cm3/mol |
| Refractive index (nD) | n1 = 2.91, n2 = 3.19 (α, hematite)[ |
| Crystal structure | Rhombohedral, hR30 (α-form)[5] |
| Space group | R3c, No. 161 (α-form)[5] |
| Point group | 3m (α-form)[5] |
| Coordination geometry | Octahedral (Fe3+, α-form, β-form)[ |


Reviews
There are no reviews yet.