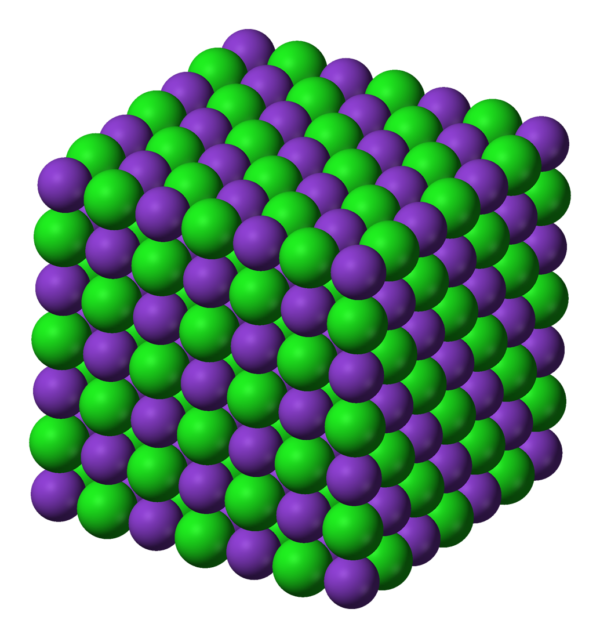
Potassium Chloride
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners (as a substitute for sodium chloride salt), and in food processing, where it may be known as E number additive E508.
It occurs naturally as the mineral sylvite, and in combination with sodium chloride as sylvinite.
Application
| Chemical formula | KCl |
|---|---|
| Molar mass | 74.555 g·mol−1 |
| Odor | odorless |
| Density | 1.984 g/cm3 |
| Melting Point | 770 °C (1,420 °F; 1,040 K) |
| Boiling Point | 1,420 °C (2,590 °F; 1,690 K) |
| Solubility in water | 27.77 g/100mL (0 °C) |
| Solubility | Soluble in glycerol, alkalies |
| Solubility in ethanol | 0.288 g/L (25 °C) |
| Acidity (pKa) | ~7 |
| Magnetic susceptibility (χ) | −39.0·10−6 cm3/mol |
| Refractive index (nD) | 1.4902 (589 nm) |


Reviews
There are no reviews yet.