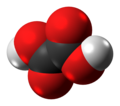
Oxalic Acid
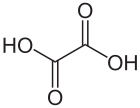
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula HO2C−CO2H, also written as (CO2H)2. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base, known as oxalate (C2O2−4), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula C2H2O4·2H2O.
Application
Used as a mordant in dyeing processes; Used in bleaches, especially for pulpwood and as cleaning agent to remove rust.
| Chemical formula | C2H2O4 |
|---|---|
| Molar mass | 90.034 g·mol−1 (anhydrous) |
| Appearance | White crystals |
| Odor | Odorless |
| Density | 1.90 g·cm3 (anhydrous, at 17 °C) |
| Melting Point | 189 to 191 °C (372 to 376 °F; 462 to 464 K) |
| Solubility in water | 46.9 g/L (5 °C), 57.2 (10 °C), 75.5 (15 °C), 95.5 (20 °C), 118 (25 °C), 139 (30 °C), 178 (35 °C), 217 (40 °C), 261 (45 °C), 315 (50 °C), 376 (55 °C), 426 (60 °C), 548 (65 °C) |
| Solubiltiy | 237 g/L (15 °C) in ethanol |
| Conjugate Base | Hydrogenoxalate |
| Magnetic susceptibility (χ) | −60.05·10−6 cm3/mol |


Reviews
There are no reviews yet.