Ortho Xylene
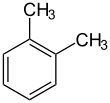
o-Xylene (ortho-xylene) is an aromatic hydrocarbon with the formula C6H4(CH3)2, with two methyl substituents bonded to adjacent carbon atoms of a benzene ring (the ortho configuration). It is a constitutional isomer of m-xylene and p-xylene, the mixture being called xylene or xylenes. o-Xylene is a colourless slightly oily flammable liquid Petroleum contains about one weight percent xylenes. Most o-xylene is produced by cracking petroleum, which affords a distribution of aromatic compounds, including xylene isomers. m-Xylene is isomerized to o-xylene. Net production was approximately 500,000 tons in the year 2000.
o-Xylene is largely used in the production of phthalic anhydride, which is a precursor to many materials, drugs, and other chemicals. Related to their easy oxidation, the methyl groups are susceptible to halogenation. When treated with elemental bromine, these groups are brominated, yielding xylylene dibromide:
Application
Mixed Xylene is used as solvent in printing, rubber, and leather industries. In thinning paints and varnishes, it can be substituted for toluene where slower drying is desired. It is used in the production of plastic bottles and polyester clothing. Used as solvent in printing, rubber, and leather industries. In thinning paints and varnishes, it can be substituted for toluene where slower drying is desired.
| Chemical formula | C8H10 |
|---|---|
| Molar mass | 106.168 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.88 g/ml |
| Melting point | −24 °C (−11 °F; 249 K) |
| Boiling point | 144.4 °C (291.9 °F; 417.5 K) |
| Solubility in water | 0.02% (20°C) |
| Solubility in ethanol | very soluble |
| Solubility in diethyl ether | very soluble |
| Vapor pressure | 7 mmHg (20°C) |
| Magnetic susceptibility (χ) | -77.78·10−6 cm3/mol |
| Refractive index (nD) | 1.50545 |
| Viscosity | 1.1049 cP at 0 °C 0.8102 cP at 20 °C |




Reviews
There are no reviews yet.