
Nickel Oxide
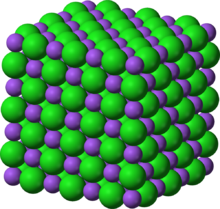
Nickel(II) oxide is the chemical compound with the formula NiO. It is the principal oxide of nickel. It is classified as a basic metal oxide. Several million kilograms are produced annually of varying quality, mainly as an intermediate in the production of nickel alloys. The mineralogical form of NiO, bunsenite, is very rare. Other nickel(III) oxides have been claimed, for example: Ni
2O 3 and NiO 2, but they have yet to be proven by X-ray crystallography.
Application
NiO has a variety of specialized applications and generally, applications distinguish between “chemical grade”, which is relatively pure material for specialty applications, and “metallurgical grade”, which is mainly used for the production of alloys. It is used in the ceramic industry to make frits, ferrites, and porcelain glazes. The sintered oxide is used to produce nickel steel alloys. Charles Édouard Guillaume won the 1920 Nobel Prize in Physics for his work on nickel steel alloys which he called invar and elinvar.
| Chemical formula | NiO |
|---|---|
| Molar mass | 74.6928 g/mol |
| Appearance | green crystalline solid |
| Density | 6.67 g/cm3 |
| Melting point | 1,955 °C (3,551 °F; 2,228 K) |
| Solubility in water | dissolves in KCN |
| Magnetic susceptibility (χ) | +660.0·10−6 cm3/mol |
| Refractive index (nD) | 2.1818 |



Reviews
There are no reviews yet.