
Methanol
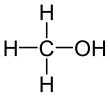
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula CH3OH (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colorless and flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). Methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide.
Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl benzoate, anisole, peroxyacids, as well as a host of more specialised chemicals.
Application
It is used as an antifreeze, solvent, fuel, and as a denaturant for ethanol. It is used in the production of fuel.
| Solubility in water | miscible |
|---|---|
| Chemical formula | CH3OH or CH4O |
| Molar mass | 32.04 g mol−1 |
| Appearance | Colourless liquid |
| Odor | Faint and similar to ethanol |
| Density | 0.792 g/cm3 |
| Melting point | −97.6 °C (−143.7 °F; 175.6 K) |
| Boiling point | 64.7 °C (148.5 °F; 337.8 K) |
| log P | −0.69 |
| Vapor pressure | 13.02 kPa (at 20 °C) |
| Acidity (pKa) | 15.5 |
| Conjugate acid | Methyloxonium |
| Conjugate base | Methanolate |
| Magnetic susceptibility (χ) | −21.40·10−6 cm3/mol |
| Refractive index (nD) | 1.33141 |
| Viscosity | 0.545 mPa·s (at 25 °C) |
| Dipole moment | 1.69 D |

Reviews
There are no reviews yet.