Light Magnesium Oxide

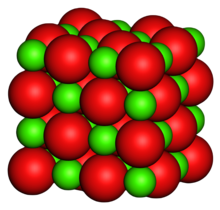
Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture.
Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from magnesia negra, a black mineral containing what is now known as manganese.
Application
It is used for refractory applications,Cattle feed, paper and pulp production, fertilizers, sorelcement and grinding wheels, brakelinings, water purification, slurry treatment, chemical application, sugar refining, rubber and plastic, glass and enamel and metallurgical application.
| Chemical formula | MgO |
|---|---|
| Molar mass | 40.304 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 3.6 g/cm3 |
| Melting point | 2,852 °C (5,166 °F; 3,125 K)[ |
| Boiling point | 3,600 °C (6,510 °F; 3,870 K)[ |
| Solubility | Soluble in acid, ammonia |
| Band gap | 7.8 eV |
| Magnetic susceptibility (χ) | −10.2·10−6 cm3/mol |
| Thermal conductivity | 45–60 W·m−1·K−1 |
| Refractive index (nD) | 1.7355 |
| Dipole moment | 6.2 ± 0.6 D |



Reviews
There are no reviews yet.