
Heptane
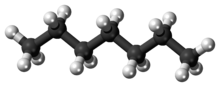 Heptane or n-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and iso-octane which is expressed as the percentage of iso-octane in heptane, and is listed on pumps for gasoline (petrol) dispensed globally.
Heptane or n-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and iso-octane which is expressed as the percentage of iso-octane in heptane, and is listed on pumps for gasoline (petrol) dispensed globally.
Heptane (and its many isomers) is widely used in laboratories as a non-polar solvent. As a liquid, it is ideal for transport and storage. In the grease spot test, heptane is used to dissolve an oil spot to show the previous presence of organic compounds on a stained paper. This is done by shaking the stained paper in a heptane solution for about half a minute.
Application
Heptane is used as a solvent in adhesives, aerosol cleaners and solvent extraction of vegetable oils and essential oils. Heptane is a component of gasoline
| Chemical formula | C7H16 |
|---|---|
| Molar mass | 100.205 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Petrolic |
| Density | 0.6795 g cm−3[3] |
| Melting point | −90.549[3] °C (−130.988 °F; 182.601 K) |
| Boiling point | 98.38[3] °C (209.08 °F; 371.53 K) |
| Solubility in water | 0.0003% (20 °C)[4] |
| log P | 4.274 |
| Vapor pressure | 5.33 kPa (at 20.0 °C) |
| Henry's law constant (kH) | 12 nmol Pa−1 kg−1 |
| Magnetic susceptibility (χ) | −85.24·10−6 cm3/mol |
| Refractive index (nD) | 1.3855[3] |
| Viscosity | 0.389 mPa·s[5] |
| Dipole moment | 0.0 D |


Reviews
There are no reviews yet.