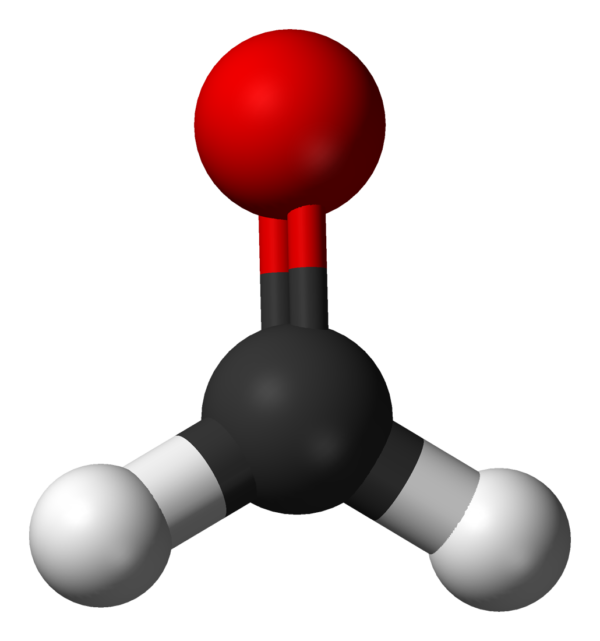
Formaldehyde
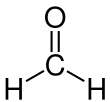
Formaldehyde (/fɔːrˈmældɪhaɪd/ ⓘ for-MAL-di-hide, US also /fər-/ ⓘ fər-) (systematic name methanal) is an organic compound with the formula CH2O and structure H−CHO. The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section Forms below). It is stored as aqueous solutions (formalin), which consists mainly of the hydrate CH2(OH)2. It is the simplest of the aldehydes (R−CHO). It is produced commercially as a precursor to many other materials and chemical compounds. In 2006, the global production rate of formaldehyde was estimated at 12 million tons per year.It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Small amounts also occur naturally.
Formaldehyde is classified as a carcinogen.[note 1] Additionally, it can cause respiratory and skin irritation upon exposure.
| Chemical formula | CH2O |
|---|---|
| Molar mass | 30.026 g·mol−1 |
| Appearance | Colorless gas |
| Density | 0.8153 g/cm3 (−20 °C)[2] (liquid) |
| Melting Point | −92 °C (−134 °F; 181 K) |
| Boiling Point | −19 °C (−2 °F; 254 K) |
| Solubility in water | 400 g/L |
| log P | 0.350 |
| Vapor Pressure | > 1 atm |
| Acidity (pKa) | 13.27 (hydrate) |
| Magnetic susceptibility (χ) | −18.6·10−6 cm3/mol |
| Dipole Moment | 2.330 D |



Reviews
There are no reviews yet.