
Ethyl Acetate
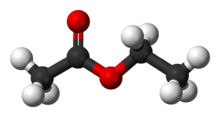 Ethyl acetate (systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula CH3CO2CH2CH3, simplified to C4H8O2. This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent.[5]
Ethyl acetate (systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula CH3CO2CH2CH3, simplified to C4H8O2. This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent.[5]
In the laboratory, mixtures containing ethyl acetate are commonly used in column chromatography and extractions.[13] Ethyl acetate is rarely selected as a reaction solvent because it is prone to hydrolysis, transesterification, and condensations.
Application
Used in glues, nail polish removers, decaffeinating tea and coffee, and cigarettes.
| Chemical formula | C4H8O2 |
|---|---|
| Molar mass | 88.106 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | nail polish-like, fruity |
| Density | 0.902 g/cm3 |
| Melting point | −83.6 °C (−118.5 °F; 189.6 K) |
| Boiling point | 77.1 °C (170.8 °F; 350.2 K) |
| Solubility in water | 8.3 g/100 mL (at 20 °C) |
| Solubility in ethanol, acetone, diethyl ether, benzene | Miscible |
| log P | 0.71[1] |
| Vapor pressure | 73 mmHg (9.7 kPa) at 20 °C[2] |
| Acidity (pKa) | 25 |
| Magnetic susceptibility (χ) | −54.10×10−6 cm3/mol |
| Refractive index (nD) | 1.3720 |
| Viscosity | 426 μPa·s (0.426 cP) at 25 °C |

Reviews
There are no reviews yet.