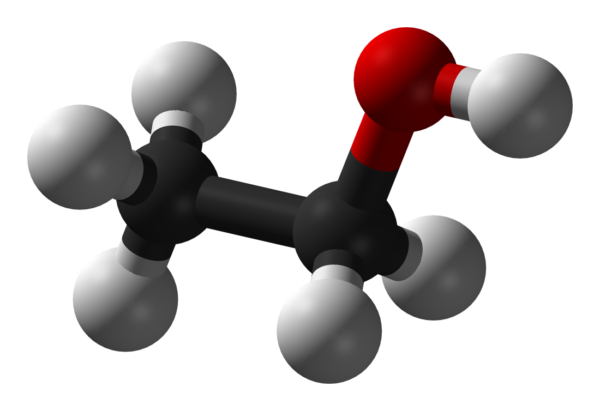
Ethanol All Grades
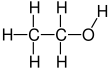
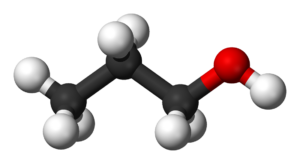
Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was 51 gigalitres (1.3×1010 US gallons), coming mostly from Brazil and the U.S.
Application
Ethanol is used as a solvent in the manufacture of varnishes and perfumes. It is used as a preservative for biological specimens; in the preparation of essences and flavorings; in many medicines and drugs.
| Chemical formula | C2H6O |
|---|---|
| Molar mass | 46.069 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | wine-like, pungent |
| Density | 0.78945 g/cm3 (at 20 °C) |
| Melting point | −114.14 ± 0.03[3] °C (−173.45 ± 0.05 °F; 159.01 ± 0.03 K) |
| Boiling point | 78.23 ± 0.09[3] °C (172.81 ± 0.16 °F; 351.38 ± 0.09 K) |
| Solubility in water | Miscible |
| log P | −0.18 |
| Vapor pressure | 5.95 kPa (at 20 °C) |
| Acidity (pKa) | 15.9 (H2O), 29.8 (DMSO) |
| Magnetic susceptibility (χ) | −33.60·10−6 cm3/mol |
| Refractive index (nD) | 1.3611 |
| Viscosity | 1.2 mPa·s (at 20 °C), 1.074 mPa·s (at 25 °C) |
| Dipole moment | 1.69 D |

Reviews
There are no reviews yet.