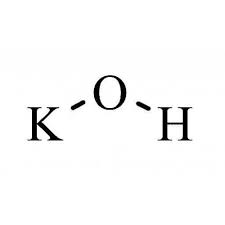
Cuastic Pottash (POTASSIUM HYDROXIDE)

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exploit its caustic nature and its reactivity toward acids. An estimated 700,000 to 800,000 tonnes were produced in 2005. KOH is noteworthy as the precursor to most soft and liquid soaps, as well as numerous potassium-containing chemicals. It is a white solid that is dangerously corrosive.
Application
” In manufacturing liquid soap, in bleaching, and in manufacturing chemicals. Potassium hydroxide is the largest-volume potassium chemical for non-fertilizer use. Potassium Hydroxide is used in chemical manufacturing including potassium carbonate and other potassium chemicals, fertilizers, phosphates, agrochemicals, alkaline batteries and dyes.
APPLICATIONS: Potassium Hydroxide is used in chemical manufacturing including potassium carbonate and other potassium chemicals, fertilizers, phosphates, agrochemicals, alkaline batteries and dyes. It is also widely used in soap and bleaching industry.”
| Chemical formula | KOH |
|---|---|
| Molar mass | 56.11 g mol−1 |
| Appearance | white solid, deliquescent |
| Odor | odorless |
| Density | 2.044 g/cm3 (20 °C)[1] |
| Melting point | 410[3][4] °C (770 °F; 683 K) |
| Boiling point | 1,327 °C (2,421 °F; 1,600 K) |
| Solubility in water | 85 g/100 mL (-23.2 °C) |
| Solubility | soluble in alcohol, glycerol |
| Solubility in methanol | 55 g/100 g (28 °C) |
| Solubility in isopropanol | ~14 g / 100 g (28 °C) |
| Acidity (pKa) | 14.7 |
| Magnetic susceptibility (χ) | −22.0·10−6 cm3/mol |
| Refractive index (nD) | 1.409 (20 °C) |


Reviews
There are no reviews yet.