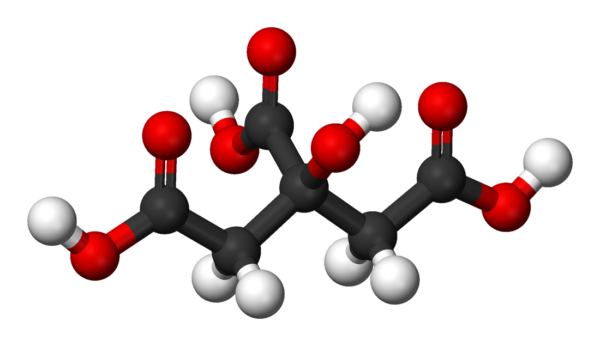
Citric Acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent.
A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as C6H5O3−7 or C3H5O(COO)3−3.
Application
Used as a flavoring and preservative in food and beverages, especially soft drinks. An excellent chelating agent, binding metals and used to remove scale from boilers and evaporators.
| Chemical formula | C6H8O7 |
|---|---|
| Molar mass | 192.123 g/mol (anhydrous), 210.14 g/mol (monohydrate) |
| Appearance | white solid |
| Density | 1.665 g/cm3 (anhydrous) |
| Melting point | 156 °C (313 °F; 429 K) |
| Boiling Point | 310 °C (590 °F; 583 K) decomposes from 175 °C |
| Solubility in water | 54% w/w (10 °C) |
| Solubility | Soluble in acetone, alcohol, ether, ethyl acetate, DMSO |
| Solubility in ethanol | 62 g/100 g (25 °C) |
| Solubility in amyl acetate | 4.41 g/100 g (25 °C) |
| Solubility in diethyl ether | 1.05 g/100 g (25 °C) |
| Solubility in 1,4-dioxane | 35.9 g/100 g (25 °C) |
| log P | -1.64 |
| Acidity (pKa) | pKa1 = 3.13[5] |
| Refractive index (nD) | 1.493–1.509 (20 °C)[4] |
| Viscosity | 6.5 cP (50% aq. sol.) |


Reviews
There are no reviews yet.