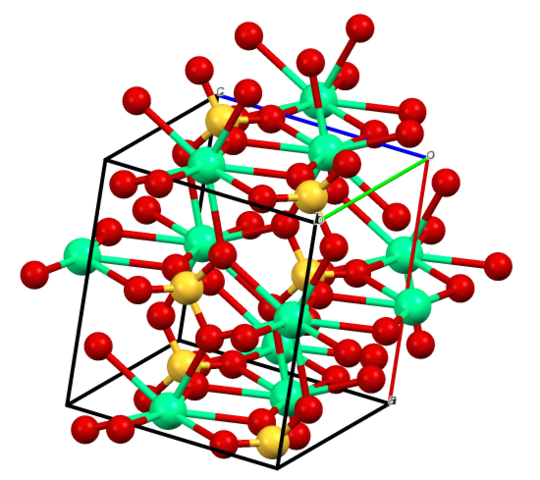
Calcium Sulphate
Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water.Calcium sulfate causes permanent hardness in water.
| Chemical formula | CASO4 |
|---|---|
| Molar mass | 136.14 g/mol (anhydrous) |
| Appearance | white solid |
| Odor | odorless |
| Density | 2.96 g/cm3 (anhydrous) |
| Melting point | 1,460 °C (2,660 °F; 1,730 K) (anhydrous) |
| Solubility in water | 0.26 g/100ml at 25 °C (dihydrate)[1] |
| Solubility product (Ksp) | 4.93 × 10−5 mol2L−2 (anhydrous) |
| Solubility in glycerol | slightly soluble (dihydrate) |
| Acidity (pKa) | 10.4 (anhydrous) |
| Magnetic susceptibility (χ) | -49.7·10−6 cm3/mol |


Reviews
There are no reviews yet.