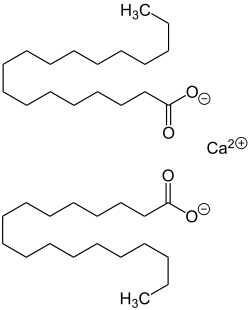
Calcium Stearate
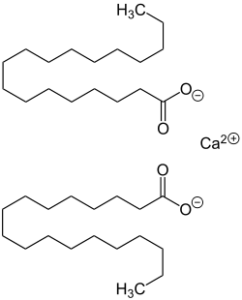
Calcium stearate is produced by heating stearic acid and calcium oxide:
- 2 C17H35COOH + CaO → (C17H35COO)2Ca + H2O
It is also the main component of soap scum, a white solid that forms when soap is mixed with hard water. Unlike soaps containing sodium and potassium, calcium stearate is insoluble in water and does not lather well.[2] Commercially it is sold as a 50% dispersion in water or as a spray dried powder. As a food additive it is known by the generic E number E470.
Application
“The cement industry often uses Calcium Stearate in the liquid form to prevent secondary efflorescence. This helps in reducing the loss of the solvated salt into the atmosphere due to continuous exposure of cement to air in the atmosphere.• The paper production line also utilizes Calcium Stearate to give paper that semi-matte gloss at a lower cost and also to extend the life of the paper.• Calcium Stearate is used in the manufacture of PVC. It acts as an external lubricant in PVC processing. It promotes fusion and has a reasonable stability to heat.• Calcium Stearate is used as thickening agent for lubricating grease.
• Applications in the personal care and pharmaceutical industry include tablet mold release, anti-tack agent, and gelling agent.”
| Chemical formula | C36H70CaO4 |
|---|---|
| Molar mass | 607.030 g·mol−1 |
| Appearance | white to yellowish-white powder |
| Density | 1.08 g/cm3 |
| Melting point | 155 °C (311 °F; 428 K) |
| Solubility in water | 0.004 g/100 mL (15 °C) |
| Solubility | soluble in hot pyridine |


Reviews
There are no reviews yet.