Benzyl Per Oxide

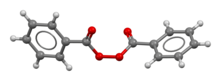
Benzoyl peroxide is a chemical compound (specifically, an organic peroxide) with structural formula (C6H5−C(=O)O−)2, often abbreviated as (BzO)2. In terms of its structure, the molecule can be described as two benzoyl (C6H5−C(=O)−, Bz) groups connected by a peroxide (−O−O−). It is a white granular solid with a faint odour of benzaldehyde, poorly soluble in water but soluble in acetone, ethanol, and many other organic solvents. Benzoyl peroxide is an oxidizer, which is principally used as in the production of polymers.
Benzoyl peroxide is mainly used in production of plastics and for bleaching flour, hair, plastics and textiles.
As a bleach, it has been used as a medication and a water disinfectant. In specialized contexts, the name may be abbreviated as BPO
Application
It is used as a bleaching agent for certain foods, an oxidizing agent, a polymerizing initiator in the manufacture of plastics, a curing agent for silicone rubber, a constituent of ointments for skin disorders, and an ingredient in various industrial processes. Benzoyl peroxide has a long history of use in the food industry as a bleaching agent added for flour, whey, and milk for cheese making. A premix of 32% benzoyl peroxide and 68% cornstarch is used in bleaching flour. The maximum amount used as a flour bleaching agent is 50 mg/kg. Benzoyl peroxide has been evaluated in the 7th JECFA meeting to an unconditional acceptance zone at 0 – 40 mg/kg and conditional acceptance level of 40 – 75 mg/kg for treatment of flour to be consumed by man (WHO, 1964). It has been reported that benzoyl peroxide is typically used in the cheese manufacture.
| Formula | C14H10O4 |
|---|---|
| Molar mass | 242.230 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| Density | 1.334 g/cm3 |
| Melting point | 103 to 105 °C (217 to 221 °F) decomposes |
| Solubility in water | poor mg/mL (20 °C) |



Reviews
There are no reviews yet.