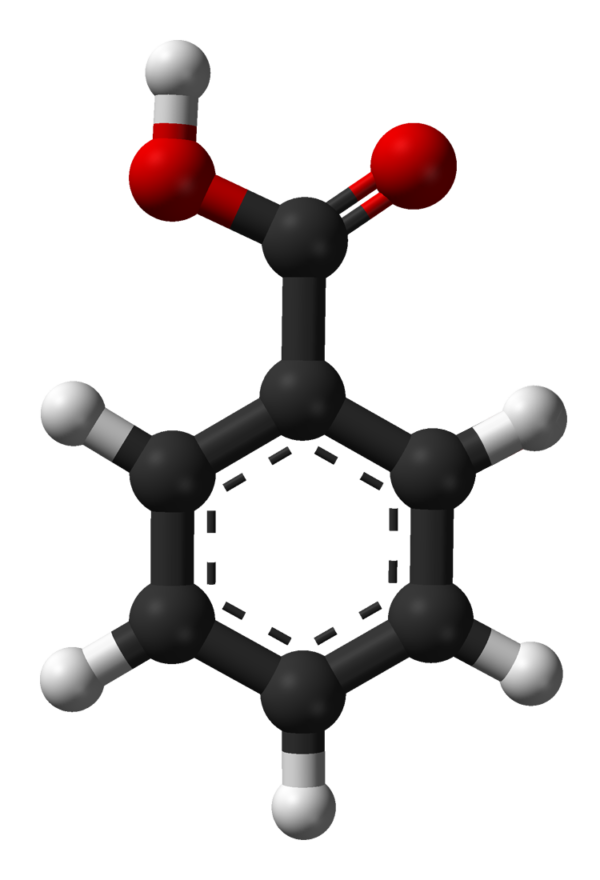
Benzoic Acid
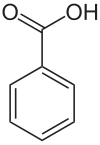
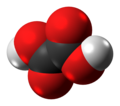
Benzoic acid /bɛnˈzoʊ.ɪk/ is a white (or colorless) solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring (C6H6) with a carboxyl (−C(=O)OH) substituent. The benzoyl group is often abbreviated “Bz” (not to be confused with “Bn” which is used for benzyl), thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –C6H5CO. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source.
Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates /ˈbɛnzoʊ.eɪt/.
Application
use of preservative application in foods, drugs and personal products.The industrial applications are as a corrosion inhibitor, as an additive to automotive engine antifreeze coolants and in other waterborne systems, as a nucleating agents for polyolefin, as a dye intermediate, as a stabilizer in photographic processing and as a catalyst
| Chemical formula | C7H6O2 |
|---|---|
| Molar mass | 122.123 g/mol |
| Appearance | Colorless crystalline solid |
| Odor | Faint, pleasant odor |
| Density | 1.2659 g/cm3 (15 °C) |
| Melting Point | 122 °C (252 °F; 395 K) |
| Boiling Point | 250 °C (482 °F; 523 K) |
| Solubility in water | 1.7 g/L (0 °C) |
| Solubility | Soluble in acetone, benzene, CCl4, CHCl3, alcohol, ethyl ether, hexane, phenyls, liquid ammonia, acetates |
| Solubility in methanol | 30 g/100 g (−18 °C) |
| Solubility in ethanol | 25.4 g/100 g (−18 °C) |
| Solubility in acetone | 54.2 g/100 g (20 °C) |
| Solubility in olive oil | 4.22 g/100 g (25 °C) |
| Solubility in 1,4-dioxane | 55.3 g/100 g (25 °C) |
| log P | 1.87 |
| Vapor pressure | 0.16 Pa (25 °C) |
| Acidity (pKa) | 4.202 (H2O) |
| Magnetic susceptibility (χ) | −70.28·10−6 cm3/mol |
| Refractive index (nD) | 1.5397 (20 °C) |
| Viscosity | 1.26 mPa (130 °C) |


Reviews
There are no reviews yet.