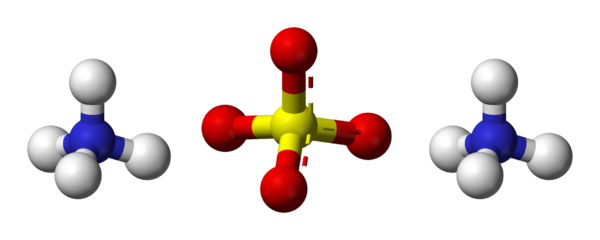
Ammonium Sulphate
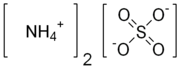
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.
Application
Catalyst to make the food dark reddish brown; Used to take off the dust on leather; Build chemical industry; Electroplating;
| Chemical formula | (NH4)2SO4 |
|---|---|
| Molar mass | 132.14 g/mol |
| Appearance | Fine white hygroscopic granules or crystals |
| Density | 1.77 g/cm3 |
| Melting point | 235 to 280 °C (455 to 536 °F; 508 to 553 K) (decomposes) |
| Solubility in water | 70.6 g per 100 g water (0 °C) |
| Solubility | Insoluble in acetone, alcohol and ether |
| Magnetic susceptibility (χ) | −67.0×10−6 cm3/mol |
| Critical relative humidity | 79.2% (30 °C) |


Reviews
There are no reviews yet.