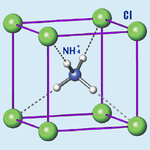
Ammonium Chloride
Ammonium chloride is an inorganic compound with the formula NH4Cl and a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form, it is known as sal ammoniac. The mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. It is also found around some types of volcanic vents. It is mainly used as fertilizer and a flavouring agent in some types of liquorice. It is the product from the reaction of hydrochloric acid and ammonia.
Application
Electrolyte in dry cells; pickling agent in zinc coating and tinning; Soldering fluxes to remove oxide coatings from metals, Improving the adhesion of the solders.
| Chemical formula | ClH4N |
|---|---|
| Molar mass | 53.49 g·mol−1 |
| Appearance | White solid, hygroscopic |
| Odor | Odorless |
| Density | 1.519 g/cm3 |
| Melting Point | 338 °C (640 °F) |
| Sublimation conditions | Decomposes at 337.6 °C at 1 atm |
| Solubility in water | 244 g/L (−15 °C) |
| Solubility product (Ksp) | 30.9 (395 g/L) |
| Solubility | Soluble in liquid ammonia, hydrazine, |
| Solubility in methanol | 32 g/kg (17 °C) |
| Solubility in ethanol | 6 g/L (19 °C) |
| Solubility in glycerol | 97 g/kg |
| Solubility in sulfur dioxide | 0.09 g/kg (0 °C) |
| Solubility in acetic acid | 0.67 g/kg (16.6 °C) |
| Vapor pressure | 133.3 Pa (160.4 °C)[7] |
| Acidity (pKa) | 9.24 |
| Magnetic susceptibility (χ) | -36.7·10−6 cm3/mol |
| Refractive index (nD) | 1.642 (20 °C) |

Reviews
There are no reviews yet.