
Aluminium Oxide
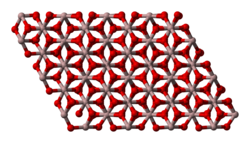
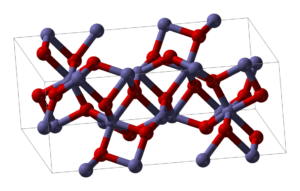
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point
Application
Aluminium oxide is used in the coating of titanium oxide, a compound that is used as a pigment for paints and plastic papers, Aluminium oxide is used to the manufacture of refractories because of its low reactivity with acids and its high boiling point, Aluminium oxide is used in the ceramic industry as an insulating material.
| Chemical formula | Al2O3 |
|---|---|
| Molar mass | 101.960 g·mol−1 |
| Appearance | white solid |
| Odor | odorless |
| Density | 3.987 g/cm3 |
| Melting point | 2,072 °C (3,762 °F; 2,345 K) |
| Boiling point | 2,977 °C (5,391 °F; 3,250 K) |
| Solubility in water | insoluble |
| Solubility | insoluble in all solvents |
| log P | 0.31860 |
| Magnetic susceptibility (χ) | −37.0×10−6 cm3/mol |
| Thermal conductivity | 30 W·m−1·K−1 |
| Refractive index (nD) | nω = 1.768–1.772 |


Reviews
There are no reviews yet.