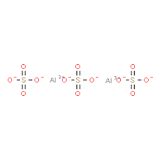
Aluminium Sulphate

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.
The anhydrous form occurs naturally as a rare mineral millosevichite, found for example in volcanic environments and on burning coal-mining waste dumps. Aluminium sulfate is rarely, if ever, encountered as the anhydrous salt. It forms a number of different hydrates, of which the hexadecahydrate Al2(SO4)3·16H2O and octadecahydrate Al2(SO4)3·18H2O are the most common. The heptadecahydrate, whose formula can be written as [Al(H2O)6]2(SO4)3·5H2O, occurs naturally as the mineral alunogen.
Application
One of the most important uses of Aluminium sulphate is in water treatment and purification, Another one of the many uses of aluminium sulphate is in dyeing and printing on cloth, aluminium sulphate was used in making paper, Other interesting uses of aluminium sulphate around the house are in gardening.
| Chemical formula | Al2(SO4)3 |
|---|---|
| Molar mass | 342.15 g/mol (anhydrous) |
| Appearance | white crystalline solid |
| Density | 2.672 g/cm3 (anhydrous) |
| Melting point | 770 °C (1,420 °F; 1,040 K) (decomposes, anhydrous) |
| Solubility in water | 31.2 g/100 mL (0 °C) |
| Solubility | slightly soluble in alcohol, dilute mineral acids |
| Acidity (pKa) | 3.3–3.6 |
| Magnetic susceptibility (χ) | −93.0×10−6 cm3/mol |
| Refractive index (nD) | 1.47[1] |




Reviews
There are no reviews yet.