Acetone
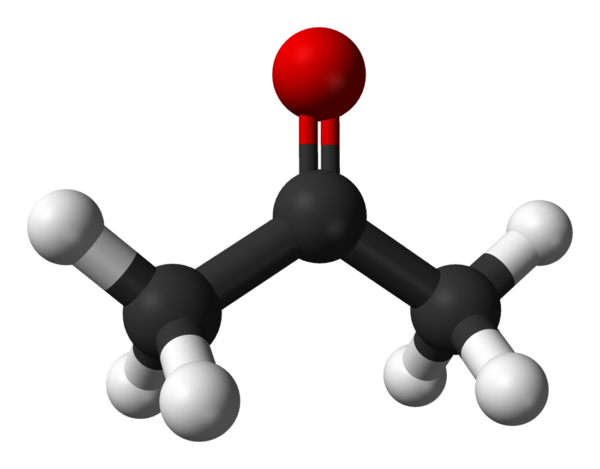
Acetone (2-propanone or dimethyl ketone) is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone (>C=O). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor.
Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and for production of methyl methacrylate and bisphenol A, which are precursors to widely-used plastics. It is a common building block in organic chemistry. It serves as a solvent in household products such as nail polish remover and paint thinner. It has volatile organic compound (VOC)-exempt status in the United States.
Acetone is produced and disposed of in the human body through normal metabolic processes. It is normally present in blood and urine. People with diabetic ketoacidosis produce it in larger amounts. Ketogenic diets that increase ketone bodies (acetone, β-hydroxybutyric acid and acetoacetic acid) in the blood are used to counter epileptic attacks in children who suffer from refractory epilepsy.
| Chemical formula | C3H6O |
|---|---|
| Molar mass | 58.080 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Pungent, fruity |
| Density | 0.7845 g/cm3 (25 °C) |
| Melting Point | −94.9 °C (−138.8 °F; 178.2 K) |
| Boiling Point | 56.08 °C (132.94 °F; 329.23 K) |
| Solubility in water | Miscible |
| Solubility | Miscible in benzene, diethyl ether, methanol, chloroform, ethanol |
| Log P | −0.24 |
| Vapor pressure | 9.39 kPa (0 °C) |
| Acidity (pKa) | 19.16 (H2O) |
| Magnetic susceptibility (χ) | −33.8·10−6 cm3/mol |
| Thermal conductivity | 0.161 W/(m·K) (25 °C)[15] |
| Refractive index (nD) | 1.3588 (20 °C) |
| viscosity | 0.306 mPa·s (25 °C) |


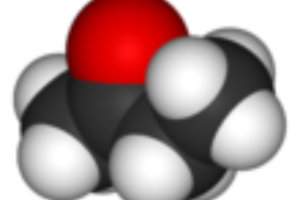
Reviews
There are no reviews yet.